University certificate
The world's largest faculty of medicine”
Why study at TECH?
Research on Clinical Infectious Diseases and Antibiotic Therapy is essential in order to achieve the most effective treatments which will improve the health of patients”
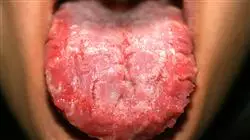
Infectious diseases have a high morbidity rate worldwide. Around 17.3 million people died from infections in 2016, with the most common causes of death being from lower respiratory infections (3.7 million), malaria (2.2 million), tuberculosis (1.3 million), diarhhea (1.3 million) and HIV/AIDS (1.1 million). In addition, the emergence of the recent COVID-19 infection, which became a pandemic in 2020, has created global chaos, with the world's leading research countries scrambling to develop effective vaccines that have been developed in just a few months.
The most important factors to take into consideration in relation to infectious diseases are demographics and human behavior, technological and industrial development, economic development and variations in land use, intercontinental travelling and commerce, climate change, microbiotic adaptation and finally the disappearance or reduction of efficient public health measures. These factors, interacting with each other, have meant that we should not consider any part of the planet reasonably isolated from the rest, nor consider that the appearance, reappearance or dissemination of imported or apparently eradicated infectious diseases in our environment is impossible.
For this reason, studies for the prevention, diagnosis, treatment and follow-up of this type of disease are constantly taking place at an international level, with antimicrobials being the key to achieving the survival of patients. However, the irrational use of these drugs has harmed their results, allowing the emergence of antimicrobial resistances that impair patient recovery. In fact, antimicrobial resistance is currently one of the greatest threats to global public health, and if urgent action is not taken, the so-called "post-antibiotic era" could be reached, where no antimicrobial would have any place in treatment and infections would be fatal. Therefore, although resistance is a natural phenomenon, the irrational use of these drugs is speeding up the process.
With this Advanced master’s degree in Clinical Infectious Diseases and Antibiotic Therapy, TECH wants to offer physicians superior specialist education, different from what they can find in any other university, and of great academic value, by uniting the most relevant specialist knowledge on clinical infectious diseases and the main advances in antibiotic therapy and antibiotic resistance. Undoubtedly, a unique academic program that not only stands out for the quality of its content, but also for its teaching team. It is composed of professionals in the area who have extensive experience in the field, and in teaching, and who are qualified to work with the latest educational technology.
We offer you a quality specialization with which you can expand your skills in the field of infectious diseases, and that will be very useful in your daily practice"
This Advanced master’s degree in Clinical Infectious Diseases and Antibiotic Therapy contains the most complete and up-to-date academic course on the university scene. The most important features of the program include:
- The latest technology in e-learning software
- A highly visual teaching system supported by graphic and schematic contents that are easy to assimilate and understand
- The development of practical case studies presented by practising experts
- State-of-the-art interactive video systems
- Teaching supported by telepractice
- Continuous updating and retraining systems
- Self-organized learning which makes the course completely compatible with other commitments
- Practical exercises for self-assessment and learning verification
- Support groups and educational synergies: Questions to the expert, discussion forums and knowledge
- Communication with the teacher and individual reflection work
- Content that is accessible from any fixed or portable device with an Internet connection
- Supplementary documentation databases are permanently available, even after the program
A high-level scientific program, supported by advanced technological development and the teaching experience of the best professionals" z
Our teaching staff is made up of practicing professionals. In this way TECH makes sure to offer the educational update of knowledge that it intends to achieve. A multidisciplinary team of professionals with training and experience in different environments, who will develop the theoretical knowledge in an efficient way, but above all, they will contribute their practical knowledge from their own experience to the course.
The effectiveness of the methodological design of this Advanced master’s degree enhances the student's understanding of the content. Developed by a multidisciplinary team of e-learning experts, it integrates the latest advances in educational technology. In this way, the student will be able to study with a range of comfortable and versatile multimedia tools that will give them the operability they need in their learning.
The design of this program is based on Problem-Based Learning: an approach that approaches learning as a highly practical process. To achieve this remotely, we will use telepractice. With the help of an innovative interactive video system and Learning from an Expert, you will be able to acquire the knowledge as if you were actually dealing with the scenario you are learning about. A concept that will allow you to integrate and consolidate learning in a more realistic and permanent way.
Access all the content of this Advanced Master’s Degree at any time. All you need is a computer or mobile device with an Internet connection"
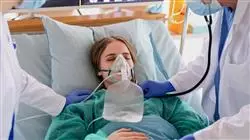
With our innovative methodology, you will be able to practice with simulated cases as if you were confronting real situations. In this way you will acquire the confidence you need to carry out your daily work"
Syllabus
The contents of this Advanced master’s degree have been developed by the different experts on this course, with a clear purpose: to ensure that our students acquire each and every one of the skills required to become true experts in this field. The content of this Advanced master’s degree will allow you to learn all aspects of the different disciplines involved in this area. A complete and well-structured program that will take you to the highest standards of quality and success.
Our academic program will allow you to aquire the necessary skills for your personal and professional development”
Module 1. Epidemiology, the Clinical Method and Scientific Research in Infectious Diseases
1.1. The Clinical Method in the Diagnostic Process of Infectious Diseases
1.1.1. Fundamental Concepts of the Clinical Method: Stages, Principles
1.1.2. The Clinical Method and its Usefulness in Infectology
1.1.3. Most Common Errors in the Application of the Clinical Method
1.2. Epidemiology in the Study of Infectious Diseases
1.2.1. Epidemiology as a Science
1.2.2. The Epidemiological Method
1.2.3. Epidemiology Tools Applied in the Study of Infectious Diseases
1.3. Clinic Epidemiology and Scientific Evidence-Based Medicine
1.3.1. Scientific Evidence and the Clinical Experience
1.3.2. The Importance of Evidence-Based Medicine in Diagnosis and Treatment
1.3.3. Clinical Epidemiology as a Powerful Weapon of Medical Thinking
1.4. Behavior of Infectious Diseases in the Population
1.4.1. Endemic
1.4.2. Epidemic
1.4.3. Pandemic
1.5. Confronting Epidemic Outbreaks
1.5.1. Diagnosis of Epidemic Outbreaks
1.5.2. Measures for the Control of Epidemic Outbreaks
1.6. Epidemiological Monitoring
1.6.1. Types of Epidemiological Monitoring
1.6.2. Designs of an Epidemiological Monitoring System
1.6.3. Usefulness and Importance of Epidemiological Monitoring
1.7. International Health Regulations
1.7.1. Components of International Health Regulations
1.7.2. Diseases Subject to International Sanitary Control
1.7.3. Importance of International Health Regulations
1.8. Mandatory Reporting Systems for Infectious Diseases
1.8.1. Characteristics of Diseases Subject to Mandatory Reporting
1.8.2. Role of the Doctor in Mandatory Reporting Systems for Infectious Diseases
1.9. Vaccines
1.9.1. Immunological Basis of Vaccines
1.9.2. Development and Production of Vaccines
1.9.3. Diseases Preventable with Vaccines
1.9.4. Experiences and Results of the Vaccine System in Cuba
1.10. Research Methodology in the Field of Health
1.10.1. The importance of Public Health in Research Methodology as a Science
1.10.2. Scientific Thought in Healthcare
1.10.3. The Scientific Method
1.10.4. Stages of Scientific Research
1.11. Information Management and the Use of New Information and Communication Technologies (ICT)
1.11.1. The Use of New ICT in the Management of Knowledge for Healthcare Professionals in the Professional Clinical, Teacher and Research Work
1.11.2. Information Literacy
1.12. Design of Research Studies for Infectious Diseases
1.12.1. Types of Studies in Healthcare and Medical Sciences
1.12.2. The Design of Research Applied to Infectious Diseases
1.13. Descriptive and Inferential Statistics
1.13.1. Summary Measures for the Different Variables in Scientific Research
1.13.2. Central Tendency Measures: Mean, Mode and Median
1.13.3. Dispersion Measures: Variants and Standard Deviation
1.13.4. Statistical Estimation
1.13.5. Population and Sample
1.13.6. Tools for Inferential Statistics
1.14. Design and Use of Databases
1.14.1. Types of Databases
1.14.2. Programs and Statistical Packages for the Management of Databases
1.15. Protocol of Scientific Research
1.15.1. Protocol Components of Scientific Research
1.15.2. Usefulness of Protocol of Scientific Research
1.16. Clinical Trials and Meta Analysis
1.16.1. Types of Clinical Trials
1.16.2. The Role of a Clinical Trial in Healthcare Research
1.16.3. Meta Analysis: Conceptual Definitions and their Methodological Design
1.16.4. Application of Meta-Analyses and their Role in the Medical Sciences
1.17. Critical Reading of Research Results
1.17.1. Medical Journals, their Role in the Dissemination of Scientific Information
1.17.2. Medical Journals of High-Impact on a Global Level in the Field of Infectology
1.17.3. Methodological Tools for Critical Reading of Scientific Literature
1.18. Publication of Scientific Research Results
1.18.1. The Scientific Article
1.18.2. Types of Scientific Articles
1.18.3. Methodology Requirements for the Publication of Scientific Research Results
1.18.4. The Process of Scientific Publications in Medical Journals
Module 2. Microbiological Diagnosis and Other Examinations for Infectious Diseases
2.1. Organization, Structure and Functioning of the Microbiology Laboratory
2.1.1. Organization and Structure of the Microbiology Laboratory
2.1.2. Functioning of a Microbiology Laboratory
2.2. Principles of the Use of Microbiological Examinations in Patients with Infectious Pathologies: The Process of Collecting Specimens
2.2.1. The Role of Microbiological Studies in the Diagnosis of Infectious Diseases
2.2.2. The Microbiological Sampling Process: Preanalytical, Analytical, and Postanalytical
2.2.3. Sampling Requirements for the Main Microbiological Studies used in Daily Clinical Practice: Blood, Urine, Stool, Sputum
2.3. Virological Studies
2.3.1. Types of Virus and their General Characteristics
2.3.2. General Characteristics of Virological Studies
2.3.3. Viral Culture
2.3.4. Viral Genome Studies
2.3.5. Studies of Antigens and Antibodies Against the Virus
2.4. Bacteriological Studies
2.4.1. Classification of Bacteria
2.4.2. General Characteristics of Bacteriological Studies
2.4.3. Stains for Bacterial Identification
2.4.4. The Study of Bacterial Antigens
2.4.5. Cultivation Methods: General and Specific
2.4.6. Bacteria That Need Special Study Methods
2.5. Mycological Studies
2.5.1. Classification
2.5.2. Main Mycological Studies
2.6. Parasitic Studies
2.6.1. Classification of Parasites
2.6.2. Studies for Protozoa
2.6.3. Studies for Helminths
2.7. Appropriate Interpretation of Microbiological Studies
2.7.1. The Microbiological Clinical Interrelationship for the Interpretation of Microbiological Studies
2.8. Interpreted Reading of the Antibiogram
2.8.1. Traditional Interpretation of the Antibiogram With Relation to the Sensitivity and Resistance to Antimicrobials
2.8.2. Interpreted Reading of the Antibiogram: Current Paradigm
2.9. Use of Microbial Map of an Institution
2.9.1. What is a Microbial Map of an Institution?
2.9.2. Clinical Application of the Microbial Map
2.10. Biosafety
2.10.1. Conceptual Definitions of Biosafety
2.10.2. Importance of Biosafety for Health Services
2.10.3. Universal Measures of Precaution
2.10.4. Manage the Biological Waste in a Healthcare Institution
2.11. The Clinical Laboratory in the Study of Infectious Diseases
2.11.1. Reactants of the Acute Phase
2.11.2. Studies of Liver Function, Internal Environment, Coagulation and Renal Function in Sepsis
2.11.3. Study of Inflammatory Liquids in the Diagnosis of Infections
2.11.4. Biomarkers Usefulness in Clinical Practice
2.12. Imaging Studies for the Diagnosis of Infectious Pathology
2.12.1. The Role of Imaging Studies in the Diagnosis of Infectious Diseases
2.12.2. The Role of Ultrasound in the Integral Evaluation of a Patient with Sepsis
2.13. The Role of Genetic and Immunological Studies
2.13.1. Studies of Genetic Illnesses and their Predisposition of Infectious Diseases
2.13.2. Immunological Studies on Immunosuppressed Patients
2.14. Usefulness of Pathological Anatomy Studies
2.14.1. Alterations in Cytological Studies According to the Type of the Biological Agent
2.14.1. Necropsy and Its Importance in Infectious Mortality
2.15. Assessment of the Severity of Infectious Diseases
2.15.1. Prognosis Scales in the Care of Patients with Infectious Pathologies Based on Laboratory Studies and Clinical Elements
2.15.2. SOFA Score Usefulness in the Current Day: Components of SOFA, What it Measures Usefulness in the Assessment of a Patient
2.15.3. Main Complications in Infectious Diseases
2.16. Worldwide Campaign Against Sepsis
2.16.1. Emergence and Evolution
2.16.2. Objectives
2.16.3. Recommendations and Impact
2.17. Bioterrorism
2.17.1. Principle Infectious Agents Used in Bioterrorism
2.17.2. International Regulations on the Management of Biological Samples
Module 3. The Immune System in Infections in the Immunosuppressed Host
3.1. Structure and Development of the Immune System
3.1.1. Composition and Development of the Immune System
3.1.2. Immune System Organs
3.1.3. Immune System Cells
3.1.4. Chemical Mediators in the Immune System
3.2. The Immune Response to Viral and Bacterial Infections
3.2.1. Main Cells Implicated in the Immune Response to Viruses and Bacteria
3.2.2. Main Chemical Mediators
3.3. The Immune Response to Mycotic and Parasitic Infections
3.3.1. Immune Response Against Filamentous and Yeast Fungi
3.3.2. Immune Response Against Protozoas
3.3.3. Immune Response Against Helminths
3.4. Most Common Clinical Manifestations of Immunosuppression
3.4.1. Types of Immunosuppression
3.4.2. Clinical Manifestations According to the Infectious Agent
3.4.3. Frequent Infections According to the Type of Immunosuppression
3.4.4. Common Infections in Immunosuppressed Patients According to the Organ System Affected
3.5. The Fever Syndrome in Neutropenic Patients
3.5.1. Most Common Clinical Manifestations
3.5.2. Most Diagnosed Infectious Agents
3.5.3. Most-Used Complementary Studies in the Integral Evaluation of a Neutropenic Fever Patient
3.5.4. Therapeutic Recommendations
3.6. Management of an Immunosuppressed Patient with Sepsis
3.6.1. Evaluation of Diagnosis, Prognosis and Treatment According to the Latest International Recommendations Endorsed by Scientific Evidence
3.7. Immunomodulatory and Immunosuppressive Therapy
3.7.1. Immunomodulators and their Clinical Use
3.7.2. Immunosuppressors and their Relation to Sepsis
Module 4. General Elements of Infectious Diseases
4.1. General and Basic Concepts of the Infectious Health-Illness Process
4.1.1. The Stages of the Infectious Process
4.1.2. The Systemic Inflammatory Response
4.1.3. Sepsis
4.1.4. Complications of Sepsis
4.2. Most Common Signs and Symptoms in Patients with Infectious Diseases
4.2.1. Local Signs and Symptoms of Sepsis
4.2.2. Systemic Signs and Symptoms of Sepsis
4.3. Main Infectious Syndromes
4.3.1. Systemic Syndromes
4.3.2. Local Syndromes
4.4. Fever of Unknown Origin (FUO)
4.4.1. Classis FUO
4.4.2. Nosocomial FUO
4.4.3. FUO in an Immunosuppressed Patient
4.4.4. FUO in HIV Infections
4.5. Fever and Rash
4.5.1. Types of Rashes
4.5.2. Main Infectious Agents Which Produce Rashes
4.6. Fever and Adenomegaly
4.6.1. Characteristics of Infectious Adenomegalies
4.6.2. Infections and Localized Adenomegalies
4.6.3. Infections and Generalized Adenomegalies
4.7. Sexually Transmitted Infections (STI)
4.7.1. Epidemiology of the STI
4.7.2. Main Agents in Sexual Transmission
4.7.3. Syndromic Approach to STIs
4.8. Septic Shock
4.8.1. Epidemiology
4.8.2. Pathophysiology
4.8.3. Clinical Manifestations and Differential Masks from the Other Types of Shock
4.8.4. Diagnosis and Evaluation of the Severity and Complications
4.8.5. Therapeutic Behavior
Module 5. Viral and Antiviral Diseases
5.1. Principles of Virology
5.1.1. Epidemiology of Viral Infections
5.1.2. Fundamental Concepts in the Study of Viruses and their Diseases
5.1.3. Main Viruses Which Affect Humans
5.2. Hemorrhagic Viral Diseases
5.2.1. Epidemiology
5.2.2. Classification
5.2.3. African Hemorrhagic Fevers
5.2.4. South American Hemorrhagic Fevers
5.2.5. Other Hemorrhagic Fevers
5.3. Arbovirus
5.3.1. General Concepts and Epidemiology of the Arboviruses
5.3.2. Dengue
5.3.3. Yellow Fever
5.3.4. Chikungunya
5.3.5. Zika
5.3.6. Other Arboviruses
5.4. Herpetic Diseases
5.4.1. Simple Herpes
5.4.2. Shingles
5.5. Viral Exanthematous Diseases
5.5.1. Rubella
5.5.2. Measles
5.5.3. Chickenpox
5.5.4. Smallpox
5.5.5. Other Exanthematous Diseases
5.6. Viral Hepatitis
5.6.1. Non-Specified Viral Infections
5.6.2. Hepatotropic Viruses
5.6.3. Acute Viral Hepatitis
5.6.4. Chronic Viral Hepatitis
5.7. Infectious Mononucleosis
5.7.1. Epidemiology
5.7.2. Etiological Agent
5.7.3. Pathogenesis
5.7.4. Clinical Picture
5.7.5. Complications
5.7.6. Diagnosis
5.7.7. Treatment
5.8. Human Rabies
5.8.1. Epidemiology
5.8.2. Etiological Agent
5.8.3. Pathogenesis
5.8.4. Clinical Picture
5.8.5. Complications
5.8.6. Diagnosis
5.8.7. Treatment
5.9. Viral Encephalitis
5.9.1. Non-Herpetic Viral Encephalitis
5.9.2. Herpetic Viral Encephalitis
5.9.3. Slow Virus Encephalitis
5.10. Antiviral
5.10.1. General Concepts
5.10.2. Main Definitions Related to Antivirals
5.10.3. Classification
5.10.4. Mechanisms of Action
5.11. Main Antivirals for Herpes Viruses
5.11.1. Mechanisms of Action
5.11.2. Antiviral Spectrum
5.11.3. Pharmacokinetics and Pharmacodynamics
5.11.4. Dose and Presentation
5.12. Main Antivirals for Respiratory Infections
5.12.1. Mechanisms of Action
5.12.2. Antiviral Spectrum
5.12.3. Pharmacokinetics and Pharmacodynamics
5.12.4. Dose and Presentation
5.13. Main Antivirals for Hepatitis
5.13.1. Mechanisms of Action
5.13.2. Antiviral Spectrum
5.13.3. Pharmacokinetics and Pharmacodynamics
5.13.4. Dose and Presentation
Module 6. Latest Information on Coronavirus Infections
6.1. Discovery and Evolution of Coronaviruses
6.1.1. Discovery of Coronaviruses
6.1.2. Global Trends in Coronavirus Infections
6.2. Main Microbiological characteristics and Members of the Coronavirus Family
6.2.1. General Microbiological Characteristics of Coronaviruses
6.2.2. Viral Genome
6.2.3. Principal Virulence Factors
6.3. Epidemiological Changes in Coronavirus Infections from its Discovery to the Present
6.3.1. Morbidity and Mortality of Coronavirus Infections from their Emergence to the Present
6.4. The Immune System and Coronavirus Infections
6.4.1. Immunological Mechanisms Involved in the Immune Response to Coronaviruses
6.4.2. Cytokine Storm in Coronavirus Infections and Immunopathology
6.4.3. Modulation of the Immune System in Coronavirus Infections
6.5. Pathogenesis and Pathophysiology of Coronavirus Infections
6.5.1. Pathophysiological and Pathogenic Alterations in Coronavirus Infections
6.5.2. Clinical Implications of the Main Pathophysiological Alterations
6.6. Risk Groups and Transmission Mechanisms of Coronaviruses
6.6.1. Main Sociodemographic and Epidemiological Characteristics of Risk Groups Affected by Coronavirus
6.6.2. Coronavirus Mechanisms of Transmission
6.7. Natural History of Coronavirus Infections
6.7.1. Stages of Coronavirus Infection
6.8. Latest Information on Microbiological Diagnosis of Coronavirus Infections
6.8.1. Sample Collection and Shipment
6.8.2. PCR and Sequencing
6.8.3. Serology Testing
6.8.4. Virus Isolation
6.9. Current Biosafety Measures in Microbiology Laboratories for Coronavirus Sample Handling
6.9.1. Biosafety Measures for Coronavirus Sample Handling
6.10. Up-to-Date Management of Coronavirus Infections
6.10.1. Prevention Measures
6.10.2. Symptomatic Treatment
6.10.3. Antiviral and Antimicrobial Treatment in Coronavirus Infections
6.10.4. Treatment of Severe Clinical Forms
6.11. Future Challenges in the Prevention, Diagnosis, and Treatment of Coronavirus
6.11.1. Global Challenges for the Development of Prevention, Diagnostic, and Treatment Strategies for Coronavirus Infections
Module 7. HIV/AIDS Infection
7.1. Epidemiology
7.1.1. Worldwide Morbidity and by Geographical Region
7.1.2. Worldwide Mortality and by Geographical Region
7.1.3. Main Vulnerable Groups
7.2. Etiopathogenesis
7.2.1. Viral Replication Cycle
7.2.2. Immune Response to HIV
7.2.3. Sanctuary Sites
7.3. Clinical Classifications of Use
7.3.1. Clinical Stages of HIV Infection
7.3.2. Clinical and Immunological Classification of HIV Infection
7.4. Clinical Manifestations According to the Stages of the Illness
7.4.1. General Clinical Manifestations
7.4.2. Clinical Manifestations By Organs and Systems
7.5. Opportunist Illnesses
7.5.1. Minor Opportunist Illnesses
7.5.2. Major Opportunist Illnesses
7.5.3. Primary Prophylaxis of Opportunistic Infections
7.5.4. Secondary Prophylaxis of Opportunistic Infections
7.5.5. Neoplasms in the Patient with HIV Infection
7.6. Diagnosis in the HIV/AIDS Infection
7.6.1. Direct HIV Screening Methods
7.6.2. Tests for Antibodies Against HIV
7.7. Antiretroviral Treatment
7.7.1. Antiretroviral Treatment Criteria
7.7.2. Main Antiretroviral Drugs
7.7.3. Monitoring of Antiretroviral Treatment
7.7.4. Antiretroviral Treatment Failure
7.8. Integral Care for a Person Living With HIV/AIDS
7.8.1. Cuban Model for Integral Care of People Living With HIV
7.8.2. Global Experiences and WHO AIDS' Leadership in HIV/AIDS Control
Module 8. Bacterial Diseases and Antimicrobials
8.1. Principles of Bacteriology
8.1.1. Fundamental Concepts of Use in Bacteriology
8.1.2. Main Gram-Positive Bacteria and their Diseases
8.1.3. Main Gram-Negative Bacteria and their Diseases
8.2. Bacterial Skin Infections
8.2.1. Folliculitis
8.2.2. Furunculosis
8.2.3. Anthrax
8.2.4. Superficial Abscesses
8.2.5. Erysipelas
8.3. Community-Acquired Pneumonia
8.3.1. Epidemiology
8.3.2. Etiology
8.3.3. Clinical Picture
8.3.4. Diagnosis
8.3.5. Prognosis Scales
8.3.6. Treatment
8.4. TB
8.4.1. Epidemiology
8.4.2. Etiopathogenesis
8.4.3. Clinical Manifestations
8.4.4. Classification
8.4.5. Diagnosis
8.4.6. Treatment
8.5. Infections of Urinary Tract and Gynecologic Infections in Women
8.5.1. Classification
8.5.2. Etiology
8.5.3. Clinical Picture
8.5.4. Diagnosis
8.5.5. Treatment
8.6. Bacterial Meningitis
8.6.1. Immunology of the Subarachnoid Space
8.6.2. Etiology
8.6.3. Clinical Picture and Complications
8.6.4. Diagnosis
8.6.5. Treatment
8.7. Osteoarticular Infections
8.7.1. Septic Arthritis
8.7.2. Osteomyelitis
8.7.3. Infectious Myositis
8.8. Enteric and Intra-Abdominal Infections
8.8.1. Acute Gastroenteritis
8.8.2. Acute Enterocolitis
8.8.3. Primary Peritonitis
8.8.4. Secondary Peritonitis
8.9. Zoonotic Disease
8.9.1. Concept
8.9.2. Epidemiology
8.9.3. Main Zoonotic Diseases
8.9.4. Leptospirosis
8.10. Antibacterials
8.10.1. General Concepts
8.10.2. Classification
8.10.3. Mechanisms of Action for Antimicrobials
8.11. Beta-Lactams Betalactams: Penicillins and Betalactamase Inhibitors
8.11.1. Structure of the Beta-Lactam Ring
8.11.2. Penicillins: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.11.3. Beta-lactamases: Types and Action on Beta-Lactam Antibiotics
8.11.4. Main Beta-Lactamase Inhibitors
8.11.5. Uses and Therapeutic Indicators
8.11.6. Cephalosporins
8.11.7. Monobactams
8.11.8. Carbapenemics
8.12. Aminoglycosides, Tetracyclines and Glycopeptides
8.12.1. Aminoglycosides: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.12.2. Tetracyclines: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.12.3. Glycopeptides: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.13. Lincosamides Rifamycins Antifolates
8.13.1. Lincosamines: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.13.2. Rifampicin: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.13.3. Antifolates: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.14. Quinolones, Macrolides and Ketolides
8.14.1. Quinolones: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.14.2. Macrolides: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.14.3. Ketolides: Classification, Mechanisms of Action, Antimicrobial Spectrum, Pharmacokinetics, Pharmacodynamics, Dosage and Presentation
8.15. New Antibiotics for Gram-Positive Infections (Lipopeptides and Oxazolidinones)
8.15.1. Lipopeptides
8.15.2. Oxazolidinones
Module 9. Mycotic Diseases
9.1. Introduction to Mycology and Superficial Mycotic Infections
9.1.1. General Concepts Used in Mycology
9.1.2. Fundamental Characteristics of Pathogenic Fungi
9.1.3. Superficial Mycotic Infections Epidermophytosis Tinea Corporis Tinea Capitis
9.2. Deep Mycotic Infections
9.2.1. Most Frequent Deep Mycoses
9.2.2. Main Clinical Manifestations of Deep Mycosis
9.3. Cryptococcosis
9.3.1. Epidemiology
9.3.2. Etiological Agent
9.3.3. Pathogenesis
9.3.4. Clinical Picture
9.3.5. Complications
9.3.6. Diagnosis
9.3.7. Treatment
9.4. Histoplasmosis
9.4.1. Epidemiology
9.4.2. Etiological Agent
9.4.3. Pathogenesis
9.4.4. Clinical Picture
9.4.5. Complications
9.4.6. Diagnosis
9.4.7. Treatment
9.5. Aspergillosis
9.5.1. Epidemiology
9.5.2. Etiological Agent
9.5.3. Pathogenesis
9.5.4. Clinical Picture
9.5.5. Complications
9.5.6. Diagnosis
9.5.7. Treatment
9.6. Systemic Candidiasis
9.6.1. Epidemiology
9.6.2. Etiological Agent
9.6.3. Pathogenesis
9.6.4. Clinical Picture
9.6.5. Complications
9.6.6. Diagnosis
9.6.7. Treatment
9.7. Coccidioidomycosis
9.7.1. Epidemiology
9.7.2. Etiological Agent
9.7.3. Pathogenesis
9.7.4. Clinical Picture
9.7.5. Complications
9.7.6. Diagnosis
9.7.7. Treatment
9.8. Blastomycosis
9.8.1. Epidemiology
9.8.2. Etiological Agent
9.8.3. Pathogenesis
9.8.4. Clinical Picture
9.8.5. Complications
9.8.6. Diagnosis
9.8.7. Treatment
9.9. Sporotrichosis
9.9.1. Epidemiology
9.9.2. Etiological Agent
9.9.3. Pathogenesis
9.9.4. Clinical Picture
9.9.5. Complications
9.9.6. Diagnosis
9.9.7. Treatment
Module 10. Parasitic, Tropical and Anti-Parasitic Diseases
10.1. Introduction to Parasitology
10.1.1. General Concepts Used in Parasitology
10.1.2. Epidemiology of the Main Parasitosis and Tropical Diseases
10.1.3. Classification of Parasites
10.1.4. Tropical Diseases and Fever Syndrome in the Tropics
10.2. Malaria
10.2.1. Epidemiology
10.2.2. Etiological Agent
10.2.3. Pathogenesis
10.2.4. Clinical Picture
10.2.5. Complications
10.2.6. Diagnosis
10.2.7. Treatment
10.3. Diseases from Intestinal Protozoas
10.3.1. Main Intestinal Protozoa
10.3.2. Diagnosis of Intestinal Protozoa
10.3.3. Amebiosis and Giardiosis
10.4. Filarial Diseases
10.4.1. Epidemiology and the Worldwide Situation
10.4.2. Clinical Syndromes
10.4.3. Main Filarial Diseases: Wuchereria Bancrofti, Brugia malayi, Brugia timori, Onchocerca volvulus, Loa loa, Mansonella Perstans, Mansonella Streptocerca y Mansonella Ozzardi
10.5. Leishmaniosis
10.5.1. Cutaneous Leishmaniasis
10.5.2. Deep Leishmaniasis
10.6. Trypanosomiasis
10.6.1. African Trypanosomiasis
10.6.2. American Trypanosomiasis
10.7. Schistosomiasis
10.7.1. Hematobium Schistosomiasis
10.7.2. Schitosomiosis Mansoni
10.7.3. Schitosomiosis Japonicum
10.7.4. Schitosomiosis Intercalatum
10.8. Intestinal Parasitism
10.8.1. Epidemiology
10.8.2. Ascaridiosis
10.8.3. Oxiuriasis
10.8.4. Hookworm Disease and Necatoriasis
10.8.5. Trichuriasis
10.9. Taeniasis Infections
10.9.1. Intestinal Taeniasis
10.9.2. Tissue Taeniasis
10.10. Antiparasitics
10.10.1. General Concepts
10.10.2. Main Definitions Used in the Management of Antiparasitics
10.10.3. Classifications: Classifications Used by Chemical Structure, Mechanism of Action or Antiparasitic Action
10.10.4. Mechanisms of Action
10.11. Antiprotozoals
10.11.1. Classification
10.11.2. Mechanisms of Action
10.11.3. Antiparasitic Spectrum
10.11.4. Pharmacokinetics and Pharmacodynamics
10.11.5. Dose and Presentation
10.12. Antiparasitic for Helminths
10.12.1. Classification
10.12.2. Mechanisms of Action
10.12.3. Antiparasitic Spectrum
10.12.4. Pharmacokinetics and Pharmacodynamics
10.12.5. Dose and Presentation
Module 11. Nosocomial Infections Associated with Healthcare and Patient Safety
11.1. Epidemiology of Nosocomial Infections
11.1.1. Operative Site Infection: Definition Epidemiology Most Frequent Germs Therapeutic Behavior
11.1.2. Nosocomial Pneumonia and Associated Mechanical Ventilation: General Concepts Epidemiology. Risk Factors. Etiology. Diagnosis. Prevention. Most-Used Antibiotics
11.2. Infection Associated With Non-Tunneled Peripheral and Central Venous Catheters and Urinary Catheters
11.2.1. Epidemiology
11.2.2. Etiology
11.2.3. Risk factors
11.2.4. Behavior for its Diagnosis and Treatment
11.3. Clostridium Difficile Infection
11.3.1. Epidemiology
11.3.2. Risk Factors
11.3.3. Clinical Manifestations
11.3.4. Diagnosis
11.3.5. Treatment
11.4. Global Vision of the Infection in Critical Patients in the ICU
11.4.1. Epidemiology
11.4.2. Risk factors
11.4.3. Etiology
11.4.4. Prevention
11.4.5. Most-Used Antibiotics
11.5. Infections Associated With Devices Used in Medicine
11.5.1. Infections Associated with Biofilm
11.5.2. Infections From Devices Used in Orthopedics
11.5.3. Infection From Devices Used in Cardiovascular Surgery
11.5.4. Infection in Neurosurgery Devices
11.5.5. Infections of Implants and Prostheses
11.6. Universal Measures for Nosocomial Infection Control:
11.6.1. Main Measures Internationally Recommended for the Control of Nosocomial Infection
11.7. Infections Associated With Healthcare
11.7.1. Definition
11.7.2. Epidemiology
11.7.3. Etiology
11.7.4. Antimicrobials Used
Module 12. The Role of Infectologists in Health Services
12.1. Infectology and its Importance in Medical Care Within Any Specialist Field
12.1.1. The Universal Nature of Infectious Pathology in Medical Specialties
12.1.2. Mastering Antibiotic Treatment
12.2. Skills and Abilities of an Infectologist
12.2.1. Skills of an Infectologist
12.2.2. Abilities of an Infectologist
12.3. The Role of Infectologists in Health Teams
12.3.1. Functions of Infectologists in Health Teams in the Different Levels of the Health System
12.4. Infectious Disease Consultation
12.4.1. Functions of an Infectologist’s Consultation
12.4.2. Pathologies to be Consulted
12.5. Scientific Update of the Infectologist’s Medical Knowledge and the Future Challenges of Infectology
12.5.1. Self-Training
12.5.2. Training and Professional Achievement
12.5.3. Future Challenges for Infectology: The Emergence of New Diseases Antimicrobial Resistance. The Development of Vaccines and Antibiotics
Module 13. Introduction to Pharmacology and Treatment
13.1. Utility of Clinical Pharmacology
13.1.1. Concept
13.1.2. Object of Study
13.1.3. Branches of Pharmacology
13.1.4. Use of Clinical Pharmacology
13.2. Pharmacokinetics: Certainties and Contradictions in its Practical Use
13.2.1. The Dynamics of Absorption, Distribution, Metabolism, and Elimination of Drugs, Especially Antimicrobials
13.3. Pharmacodynamics: Its Use in the Practical Use of New Antimicrobials
13.3.1. Molecular Mechanisms of Action of Drugs, Especially Antimicrobials
13.3.2. Drug-Drug Interactions of Antibiotics with Other Medications
13.3.3. Pharmacokinetics/Pharmacodynamics Models in Antibiotic Use
13.4. Pharmacovigilance
13.4.1. Concept
13.4.2. Objectives
13.4.3. Antibiotic Adverse Reactions
13.5. Pharmacoepidemiology: Update on Antimicrobial Research
13.5.1. Concept
13.5.2. Objectives
13.5.3. Drug Usage Studies
13.6. Clinical Trials
13.6.1. Concept
13.6.2. Methodology
13.6.3. Objectives
13.6.4. The Stages of Clinical Trials
13.6.5. Uses
13.7. Meta-Analysis
13.7.1. Concept
13.7.2. Methodology
13.7.3. Objectives
13.7.4. Uses
13.8. Rational Treatment: From Old to New and Evidence-Based Medicine
13.8.1. Stages of Rational Treatment
13.8.2. Use and Importance of Rational Treatment
13.9. Clinical Practice Guidelines: New Approaches to Practical Application
13.9.1. Creating Clinical Practice Guidelines
13.9.2. The Impact of Clinical Practice Guidelines
13.10. Clinical Pharmacology: Advances and Future Perspectives for the Improvement of Antibiotic Treatment
13.10.1. Research Activities and Scientific Advances: Is it Pharmacy Fiction?
13.10.2. Molecular Pharmacology and its Role in Antibiotic Therapy
Module 14. Antimicrobials: General Aspects
14.1. History and Development of Antimicrobials
14.1.1. Emergence and Development of Antimicrobial Treatments
14.1.2. Impact on Morbimortality of Infectious Diseases
14.2. Classifications: Practical and Future Use of Each One
14.2.1. Chemical Classification
14.2.2. Classification by Antimicrobial Action
14.2.3. Classification According to their Antimicrobial Spectrum
14.3. Update on the Mechanisms of Action of Antimicrobials
14.3.1. Main Antimicrobial Mechanisms of Action
14.4. General and Latest Elements of Antimicrobial Treatments
14.4.1. General and Recent Concepts in the Use of Antimicrobials
14.4.2. New Developments in the Use of Antimicrobial Combinations
14.4.3. Interactions between Antimicrobials
14.5. Antibiotic Prophylaxis: Its Current Role in Surgical Morbidity and Mortality
14.5.1. Concept
14.5.2. Objectives
14.5.3. Types of Antibiotic Prophylaxis
14.5.4. Perioperative Antibiotic Prophylaxis
14.6. Phased Antibiotic Treatment: Current Criteria
14.6.1. Concept
14.6.2. Principles
14.6.3. Objectives
14.7. Latest Concepts in the Use of Antibiotics in Renal Failure
14.7.1. Renal Excretion of Antibiotics
14.7.2. Renal Toxicity of Antibiotics
14.7.3. Dose Modification in Renal Failure
14.8. Antibiotics and the Blood-Brain Barrier: Recent Findings
14.8.1. The Passage of Antibiotics through the Blood-Brain Barrier
14.8.2. Antibiotics in Central Nervous System Infections
14.9. Antibiotics and Liver Failure: Progress and Future Challenges
14.9.1. Hepatic Metabolism of Antibiotics
14.9.2. Hepatic Toxicity of Antimicrobials
14.9.3. Dose Adjustment in Hepatic Insufficiency
14.10. Antibiotic Use in the Immunosuppressed: The New Paradigm
14.10.1. Immune Response to Infection
14.10.2. Main Opportunistic Germs in the Immunosuppressed
14.10.3. Principles for the Choice and Duration of Antibiotic Therapy in the Immunosuppressed
14.11. Antibiotics in Pregnancy and Lactation: The Safety of their Use According to the Latest Scientific Findings
14.11.1. The Passage of Antibiotics through the Placenta
14.11.2. Antibiotics and Breast Milk
14.11.3. Teratogenicity of Antibiotics
Module 15. Antivirals
15.1. General Features of Antivirals
15.1.1. Classification
15.1.2. Main Indications of Antivirals
15.2. Mechanisms of Action
15.2.1. Mechanisms of Action of Antivirals
15.3. Antivirals for Hepatitis: New Recommendations and Future Research Projections
15.3.1. Specific Viral Hepatitis
15.3.2. Hepatitis B Treatment
15.3.3. Hepatitis C Treatment
15.4. Antivirals for Respiratory Infections: Current Scientific Evidence
15.4.1. Main Respiratory Viruses
15.4.2. Influenza Treatment
15.4.3. Other Respiratory System Virus Treatments
15.5. Antivirals for Herpes Viruses: Recent Changes in Management
15.5.1. Main Herpes Virus Infections
15.5.2. Herpes Simplex Infection Treatment
15.5.3. Treatment of Varicella Zoster Virus Infections
15.6. Antiretrovirals for HIV: Certainties and Controversies. Future Challenges
15.6.1. Classification of Antiretrovirals
15.6.2. Mechanisms of Action of Antiretrovirals
15.6.3. Antiretroviral Treatment of HIV Infection
15.6.4. Adverse Reactions
15.6.5. Antiretroviral Treatment Failure
15.7. Topical Antivirals
15.7.1. Main Viral Infections of the Skin and Mucous Membranes
15.7.2. Topical Antivirals
15.8. Update on Interferons: Their Use in Viral and Non-Infectious Diseases
15.8.1. Classification and Action of Interferons
15.8.2. Uses of Interferons
15.8.3. Adverse Reactions of Interferons
15.9. New Areas of Antiviral Development
15.9.1. Antibiotics in Viral Hemorrhagic Diseases
15.9.2. Future Prospects for Antiviral Chemotherapy
Module 16. Antibiotics I
16.1. Advances in the Knowledge of the Synthesis and Structure of the Beta-Lactam Ring
16.1.1. Structure of the Beta-Lactam Ring
16.1.2. Drugs that Act on the Synthesis of the Beta-Lactam Ring
16.2. Penicillins: New Drugs and their Future Role in Anti-Infection Treatments
16.2.1. Classification
16.2.2. Mechanism of Action
16.2.3. Antimicrobial Spectrum
16.2.4. Pharmacokinetics and Pharmacodynamics
16.2.5. Therapeutic Uses
16.2.6. Adverse Effects
16.2.7. Presentation and Dosage
16.3. Antistaphylococcal Penicillins: From Old to New and their Practical Implications
16.3.1. Classification
16.3.2. Mechanism of Action
16.3.3. Antimicrobial Spectrum
16.3.4. Pharmacokinetics and Pharmacodynamics
16.3.5. Therapeutic Uses
16.3.6. Adverse Effects
16.3.7. Presentation and Dosage
16.4. Antipseudomonal Penicillins: Current Resistance Challenge
16.4.1. Classification
16.4.2. Mechanism of Action
16.4.3. Antimicrobial Spectrum
16.4.4. Pharmacokinetics and Pharmacodynamics
16.4.5. Therapeutic Uses
16.4.6. Adverse Effects
16.4.7. Presentation and Dosage
16.5. Cephalosporins: Present and Future
16.5.1. Classification
16.5.2. Mechanism of Action
16.5.3. Antimicrobial Spectrum
16.5.4. Pharmacokinetics and Pharmacodynamics
16.5.5. Therapeutic Uses
16.5.6. Adverse Effects
16.5.7. Presentation and Dosage
16.6. Oral Cephalosporins: New Developments in their Outpatient Use
16.6.1. Classification
16.6.2. Mechanism of Action
16.6.3. Antimicrobial Spectrum
16.6.4. Pharmacokinetics and Pharmacodynamics
16.6.5. Therapeutic Uses
16.6.6. Adverse Effects
16.6.7. Presentation and Dosage
16.7. Monobactams
16.7.1. Classification
16.7.2. Mechanism of Action
16.7.3. Antimicrobial Spectrum
16.7.4. Pharmacokinetics and Pharmacodynamics
16.7.5. Therapeutic Uses
16.7.6. Adverse Effects
16.7.7. Presentation and Dosage
16.8. Carbapenetics
16.8.1. Classification
16.8.2. Mechanism of Action
16.8.3. Antimicrobial Spectrum
16.8.4. Pharmacokinetics and Pharmacodynamics
16.8.5. Therapeutic Uses
16.8.6. Adverse Effects
16.8.7. Presentation and Dosage
16.9. Batalactamases: The Recent Discovery of Strains and their Role in Resistance
16.9.1. Classification
16.9.2. Action on Beta-Lactams
16.10. Beta-Lactamase Inhibitors
16.10.1. Classification
16.10.2. Mechanism of Action
16.10.3. Antimicrobial Spectrum
16.10.4. Pharmacokinetics and Pharmacodynamics
16.10.5. Therapeutic Uses
16.10.6. Adverse Effects
16.10.7. Presentation and Dosage
Module 17. Antibiotics II
17.1. Glycopeptides: The New Drugs for Gram Positive Germs
17.1.1. Classification
17.1.2. Mechanism of Action
17.1.3. Antimicrobial Spectrum
17.1.4. Pharmacokinetics and Pharmacodynamics
17.1.5. Therapeutic Uses
17.1.6. Adverse Effects
17.1.7. Presentation and Dosage
17.2. Cyclic Lipopeptides: Recent Advances and its Future Role
17.2.1. Classification
17.2.2. Mechanism of Action
17.2.3. Antimicrobial Spectrum
17.2.4. Pharmacokinetics and Pharmacodynamics
17.2.5. Therapeutic Uses
17.2.6. Adverse Effects
17.2.7. Presentation and Dosage
17.3. Macrolides: Their Role as an Immunomodulator in the Respiratory System
17.3.1. Classification
17.3.2. Mechanism of Action
17.3.3. Antimicrobial Spectrum
17.3.4. Pharmacokinetics and Pharmacodynamics
17.3.5. Therapeutic Uses
17.3.6. Adverse Effects
17.3.7. Presentation and Dosage
17.4. Ketolides
17.4.1. Classification
17.4.2. Mechanism of Action
17.4.3. Antimicrobial Spectrum
17.4.4. Pharmacokinetics and Pharmacodynamics
17.4.5. Therapeutic Uses
17.4.6. Adverse Effects
17.4.7. Presentation and Dosage
17.5. Tetracyclines: Old and New Indications According to the Most Recent Advances in Emerging Diseases
17.5.1. Classification
17.5.2. Mechanism of Action
17.5.3. Antimicrobial Spectrum
17.5.4. Pharmacokinetics and Pharmacodynamics
17.5.5. Therapeutic Uses
17.5.6. Adverse Effects
17.5.7. Presentation and Dosage
17.6. Aminoglycosides: Facts and Realities of their Current and Future Utilization
17.6.1. Classification
17.6.2. Mechanism of Action
17.6.3. Antimicrobial Spectrum
17.6.4. Pharmacokinetics and Pharmacodynamics
17.6.5. Current Therapeutic Uses and Future Trends
17.6.6. Adverse Effects
17.6.7. Presentation and Dosage
17.7. Quinolones: All Generations and Practical Use
17.7.1. Classification
17.7.2. Mechanism of Action
17.7.3. Antimicrobial Spectrum
17.7.4. Pharmacokinetics and Pharmacodynamics
17.7.5. Therapeutic Uses
17.7.6. Adverse Effects
17.7.7. Presentation and Dosage
17.8. Respiratory Quinolones: Latest Recommendations on their Use
17.8.1. Classification
17.8.2. Mechanism of Action
17.8.3. Antimicrobial Spectrum
17.8.4. Pharmacokinetics and Pharmacodynamics
17.8.5. Therapeutic Uses
17.8.6. Adverse Effects
17.8.7. Presentation and Dosage
17.9. Streptogramins
17.9.1. Classification
17.9.2. Mechanism of Action
17.9.3. Antimicrobial Spectrum
17.9.4. Pharmacokinetics and Pharmacodynamics
17.9.5. Therapeutic Uses
17.9.6. Adverse Effects
17.9.7. Presentation and Dosage
Module 18. Antibiotics III
18.1. Oxazolinones
18.1.1. Classification
18.1.2. Mechanism of Action
18.1.3. Antimicrobial Spectrum
18.1.4. Pharmacokinetics and Pharmacodynamics
18.1.5. Therapeutic Uses
18.1.6. Adverse Effects
18.1.7. Presentation and Dosage
18.2. Sulfas
18.2.1. Classification
18.2.2. Mechanism of Action
18.2.3. Antimicrobial Spectrum
18.2.4. Pharmacokinetics and Pharmacodynamics
18.2.5. Therapeutic Uses
18.2.6. Adverse Effects
18.2.7. Presentation and Dosage
18.3. Lincosamides
18.3.1. Classification
18.3.2. Mechanism of Action
18.3.3. Antimicrobial Spectrum
18.3.4. Pharmacokinetics and Pharmacodynamics
18.3.5. Therapeutic Uses
18.3.6. Adverse Effects
18.3.7. Presentation and Dosage
18.4. Rifamycin: Practical Use in TB and Other Infections Today
18.4.1. Classification
18.4.2. Mechanism of Action
18.4.3. Antimicrobial Spectrum
18.4.4. Pharmacokinetics and Pharmacodynamics
18.4.5. Therapeutic Uses
18.4.6. Adverse Effects
18.4.7. Presentation and Dosage
18.5. Antifolates
18.5.1. Classification
18.5.2. Mechanism of Action
18.5.3. Antimicrobial Spectrum
18.5.4. Pharmacokinetics and Pharmacodynamics
18.5.5. Therapeutic Uses
18.5.6. Adverse Effects
18.5.7. Presentation and Dosage
18.6. Antibiotics for Leprosy: Recent Advances
18.6.1. Classification
18.6.2. Mechanism of Action
18.6.3. Antimicrobial Spectrum
18.6.4. Pharmacokinetics and Pharmacodynamics
18.6.5. Therapeutic Uses
18.6.6. Adverse Effects
18.6.7. Presentation and Dosage
18.7. Antituberculosis Drugs: Latest Recommendations for their Use
18.7.1. Classification
18.7.2. Mechanism of Action
18.7.3. Antimicrobial Spectrum
18.7.4. Pharmacokinetics and Pharmacodynamics
18.7.5. Therapeutic Uses
18.7.6. Adverse Effects
18.7.7. Presentation and Dosage
18.8. Parenteral Antibiotic Use in Outpatients: Latest Recommendations
18.8.1. Main Indications for Parenteral Antibiotics in Outpatients
18.8.2. Monitoring Outpatients Receiving Parenteral Antibiotic Treatment
18.9. The Latest on Antibiotics for Multidrug Resistant Bacteria
18.9.1. Antibiotics for Multidrug-Resistant GramPositive Bacteria
18.9.2. Antibiotics for Multidrug-Resistant GramNegative Bacteria
Module 19. Antimycotics
19.1. General Elements
19.1.1. Concept
19.1.2. Origins and Development
19.2. Classification
19.2.1. Classification According to Chemical Structure
19.2.2. Classification According to Action: Local and Systemic
19.3. Mechanisms of Action
19.3.1. Mechanisms of Action of Antifungal Agents
19.4. Systemic Antifungal Agents: News on their Toxicity and their Present and Future Indications
19.4.1. Antimicrobial Spectrum
19.4.2. Pharmacokinetics and Pharmacodynamics
19.4.3. Therapeutic Uses
19.4.4. Adverse Effects
19.4.5. Presentation and Dosage
19.5. Amphotericin B: Novel Concepts in its Use
19.5.1. Mechanism of Action
19.5.2. Antimicrobial Spectrum
19.5.3. Pharmacokinetics and Pharmacodynamics
19.5.4. Therapeutic Uses
19.5.5. Adverse Effects
19.5.6. Presentation and Dosage
19.6. Deep Mycosis Treatment: Current Events and Future Perspectives
19.6.1. Aspergillosis
19.6.2. Coccidioidomycosis
19.6.3. Cryptococcosis
19.6.4. Histoplasmosis
19.7. Local Antifungals
19.7.1. Antimicrobial Spectrum
19.7.2. Pharmacokinetics and Pharmacodynamics
19.7.3. Therapeutic Uses
19.7.4. Adverse Effects
19.7.5. Presentation and Dosage
19.8. Treatment of Skin and Mucous Mycosis
19.8.1. Tinea Capitis
19.8.2. Skin Tinea
19.8.3. Onychomycosis
19.9. Liver Toxicity of Systemic Antifungal Agents: Future Challenges
19.9.1. Liver Metabolism of Antifungal Agents
19.9.2. Hepatotoxicity of Antifungal Agents
Module 20. Antiparasitics
20.1. General Elements
20.1.1. Concept
20.1.2. Origins and Development
20.2. Classification
20.2.1. Classification by Chemical Structure
20.2.2. Classification by Action Against Different Parasites
20.3. Mechanisms of Action
20.3.1. Action Mechanisms of Antiparasitics
20.4. Antiparasitics for Intestinal Parasitism: New Advances
20.4.1. Classification
20.4.2. Mechanism of Action
20.4.3. Antimicrobial Spectrum
20.4.4. Pharmacokinetics and Pharmacodynamics
20.4.5. Therapeutic Uses
20.4.6. Adverse Effects
20.4.7. Presentation and Dosage
20.5. Antimalarials: Latest WHO Recommendations
20.5.1. Classification
20.5.2. Mechanism of Action
20.5.3. Antimicrobial Spectrum
20.5.4. Pharmacokinetics and Pharmacodynamics
20.5.5. Therapeutic Uses
20.5.6. Adverse Effects
20.5.7. Presentation and Dosage
20.6. Update on Antiparasitics for Filariasis
20.6.1. Classification
20.6.2. Mechanism of Action
20.6.3. Antimicrobial Spectrum
20.6.4. Pharmacokinetics and Pharmacodynamics
20.6.5. Therapeutic Uses
20.6.6. Adverse Effects
20.6.7. Presentation and Dosage
20.7. Latest Advances in Antiparasitics for Trypanosomiasis
20.7.1. Classification
20.7.2. Mechanism of Action
20.7.3. Antimicrobial Spectrum
20.7.4. Pharmacokinetics and Pharmacodynamics
20.7.5. Therapeutic Uses
20.7.6. Adverse Effects
20.7.7. Presentation and Dosage
20.8. Antiparasitics for Schistosomiasis
20.8.1. Classification
20.8.2. Mechanism of Action
20.8.3. Antimicrobial Spectrum
20.8.4. Pharmacokinetics and Pharmacodynamics
20.8.5. Therapeutic Uses
20.8.6. Adverse Effects
20.8.7. Presentation and Dosage
20.9. Antiparasitics for Leishmaniasis
20.9.1. Classification
20.9.2. Mechanism of Action
20.9.3. Antimicrobial Spectrum
20.9.4. Pharmacokinetics and Pharmacodynamics
20.9.5. Therapeutic Uses
20.9.6. Adverse Effects
20.9.7. Presentation and Dosage
20.10. Treatment of Other Less Common Parasitosis
20.10.1. Dracunculiasis
20.10.2. Hydatid Cyst
20.10.3. Other Tissue Parasites
Module 21. Antibiotic Resistance
21.1. Emergence and Development of Antibiotic Resistance
21.1.1. Concept
21.1.2. Classification
21.1.3. Origins and Development
21.2. Mechanisms of Antibiotic Resistance: An Update
21.2.1. Mechanisms of Antimicrobial Resistance
21.2.2. New Resistance Mechanisms
21.3. Staphylococcal Resistance: Yesterday, Today, and Tomorrow
21.3.1. Evolution of Staphylococcal Resistance
21.3.2. Mechanisms of Staphylococcal Resistance
21.4. Resistance of Gram-Positive Germs: Latest Recommendations
21.4.1. Evolution and Resistance of GramPositive Germs
21.4.2. Resistance Mechanisms of GramPositive Germs
21.5. Resistance of Gram-Negative Germs: Current Clinical Implications
21.5.1. Evolution of GramNegative Germ Resistance
21.5.2. Resistance Mechanisms of GramNegative Germs
21.6. Virus Resistance
21.6.1. Evolution of Virus Resistance
21.6.2. Virus Resistance Mechanisms
21.7. Fungal Resistance
21.7.1. Evolution of Fungal Resistance
21.7.2. Mechanisms of Fungal Resistance
21.8. Parasite Resistance: An Emerging Problem
21.8.1. Evolution of Parasite Resistance
21.8.2. Mechanisms of Parasite Resistance
21.8.3. Resistance to Antimalarials
21.9. New Mechanisms of Antibiotic Resistance and Superbugs
21.9.1. Emergence and Progression of Superbugs
21.9.2. New Resistance Mechanisms of Superbugs
21.10. Antibiotic Resistance Control Mechanisms and Programs
21.10.1. Antibiotic Resistance Control Strategies
21.10.2. Global Program and International Experiences in the Control of Antibiotic Resistance
Module 22. Monitoring and Controlling the Use of Antimicrobials
22.1. Antibiotic Treatment Duration in the Treatment of Infections: New Role of Biomarkers
22.1.1. Update on the Adequate Duration of the Most Frequent Infections
22.1.2. Clinical and Laboratory Parameters to Determine the Duration of Treatment
22.2. Antimicrobial Usage Studies: Most Recent Impacts
22.2.1. The Significance of Antimicrobial Usage Studies
22.2.2. Results of Greater Impact in Recent Years by Antimicrobial Usage
22.3. Antibiotic Committees in Hospitals: Their Role in the Future
22.3.1. Structure and Operation
22.3.2. Objectives
22.3.3. Activities
22.3.4. Impacts
22.4. Antimicrobial Use Policies: Current Impact on Antimicrobial Use
22.4.1. Concepts
22.4.2. Types of Policies
22.4.3. Objectives
22.4.4. Impacts
22.5. Pharmacotherapeutic Committees: Practical Importance
22.5.1. Structure and Function
22.5.2. Objectives
22.5.3. Activities
22.5.4. Impacts
22.6. Infectious Disease Specialists and their Role in the Rational Use of Antimicrobials
22.6.1. Functions and Activities of Infectious Disease Specialists to Promote and Encourage the Rational Use of Antimicrobials
22.7. Impact of Training and Professional Development on Antimicrobial Utilization
22.7.1. Importance of Training and Professional Development
22.7.2. Types
22.7.3. Impacts
22.8. Hospital Strategies for Rational Antimicrobial Use: What the Evidence Says
22.8.1. Hospital Strategies for the Control of the Rational Use of Antimicrobials
22.8.2. Impacts
22.9. Scientific Research for the Future Control and Monitoring of Antibiotic Therapy in Patients with Sepsis
22.9.1. Search for New Parameters and Markers for Monitoring and Control of Antibiotic Therapeutics
Module 23. Antibiotics and Antimicrobial Treatments of the Future
23.1. Research, Approval, and Commercialization of New Antibiotics
23.1.1. Antimicrobial Research
23.1.2. Antimicrobial Approval Process
23.1.3. Antimicrobial Marketing and Large Pharmaceutical Companies
23.2. Ongoing Clinical Trials for the Approval of New Antibiotics
23.2.1. New Clinical Trials on Antimicrobials
23.3. Old Antibiotics with New Uses
23.3.1. The Role of Old Antibiotics with New Uses
23.3.2. Antimicrobial Withdrawal
23.3.3. Chemical Alterations of Old Antimicrobials
23.4. Treatment Goals and New Ways to Fight Infections: What's New in Research
23.4.1. New Treatment Goals
23.4.2. New Ways to Treat Sepsis
23.5. Monoclonal Antibodies in Infections: Present and Future
23.5.1. Origin and Emergence of Monoclonal Antibodies
23.5.2. Classification
23.5.3. Clinical Uses
23.5.4. Impact Results in Infectious Diseases
23.6. Other Drugs to Regulate and Stimulate Immune Response against Infection
23.6.1. Drugs to Regulate and Control the Immune Response
23.7. Futuristic Antibiotics
23.7.1. The Future of Antimicrobials
23.7.2. Antibiotics of the Future
After completing our Advanced master’s degree, you will be able to compete in your profession at the highest professional level"
Advanced Master's Degree in Clinical Infectology and Antibiotic Therapy
The number of deaths due to infectious diseases represents a high percentage worldwide. Moreover, given the epidemiological alerts of the WHO in the face of the emergence of events that compromise public health, the development of studies for the prevention, diagnosis and treatment of these pathologies is essential. For this reason, at TECH Global University we developed the Advanced Master's Degree in Infectious Diseases and Antibiotic Therapy, a program that brings together in a complete and updated way the latest advances in this subspecialty, as well as the necessary techniques and tools so that you can participate in the process of research and development of drugs that are effective in the therapy and antibiotic resistance.
Specialize in Infectious Diseases
If you want to obtain a higher qualification in the field of Clinical Infectious Diseases and Antibiotic Therapy, our Advanced Master's Degree is the one for you. Through a curriculum designed with the highest rigor, you will delve into the functions of the care, teaching or research work of infectious diseases; you will review the most relevant concepts and topics of this discipline, including epidemiology, clinical and investigative method of pathologies; the role of the infectologist from the field of microbiology and health services; and antibiotics and antimicrobial therapies of the future. Thus, supported by the best scientific evidence, you will successfully perform and provide your services for the correct use of drugs and therapeutic methods of resistance to the spread of microorganisms, as well as infectious diseases, with a multidisciplinary and comprehensive approach that facilitates the control of these pathologies.







